
Although MannKind Corporation (MNKD) finally won its decade-long fight for FDA approval to sell its Afrezza insulin therapy system in the U.S, most investors are disappointed that the powered drug inhaler must carry a warning label that it is not a substitute for injectable insulin and that it is not recommended for individuals with asthma or a serious lung disease.
FDA approval for a new drug usually causes a big jump in manufacturers’ share price, but MNKD stock plunged by 20% to $8.20, recovered to $10, and then rose in the aftermarket trading on Friday to $11.
Valencia, California-based Mankind went public in 2004 and traded as high as $23.25 on the promise that inhaling Afrezza at the beginning of a meal or within 20 minutes of starting to eat would replace insulin injections. It has been a rocky ride for investors.
Diabetes refers to a set of diseases that cause high blood sugar levels over a prolonged period of time. Symptoms include frequent urination, increased thirst, and increased hunger. Diabetes can lead to heart disease, stroke, kidney failure, neuropathy, foot ulcers, blindness, and eventually death. Approximately 9.3% or 29.3 million Americans suffer from diabetes, according to the American Diabetes Association. For drug manufacturers, the good news is that 2/3 of individuals suffering from the disease have been diagnosed and are on some form of treatment, ranging from diet to daily insulin shots that will last for the rest of their lives.
Ten years ago, it appeared there would be a large number of different inhaled insulin delivery systems competing for a piece of the expected $21 billion annual market. But the FDA-approved Exubera by Pfizer was voluntarily withdrawn due to complications in 2007, after it was on the market for only a year. In one of the largest product failures in the history of the industry, pharmaceutical giants Novo Nordisk Sanofi-Aventis and Eli Lilly shut down clinical development programs after spending billions of dollars on unsuccessful development.
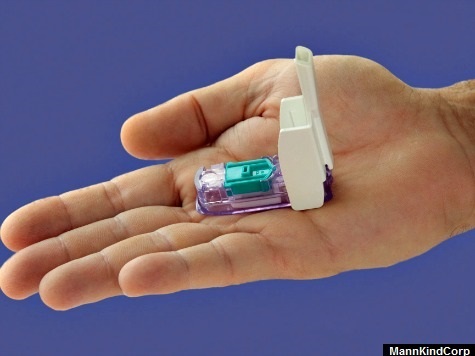
The only company that remained in clinical trials was MannKind’s Afrezza. Its whistle-sized inhaler that supposedly delivered insulin faster than injectable products such as Eli Lilly’s “Humalog” and Novo Nordisk’s “NovoLog” was expected to dominate the diabetes insulin therapy market.
Despite huge expectations by analysts and investors, the FDA on January 19, 2011 rejected approval of Afrezza and demanded the company complete two new clinical trials that were expected to take at least two more years. MNKD shares were halted from trading at $9.10, and then opened in after-hours trading down 43% at $5.15. The stock would eventually fall to $1.60 and was just $3.80 in March of this year.
The FDA’s approval of Afrezza to control Type 1 and Type 2 Diabetes in adult patients requires the company to have a warning label for possible bronchospasms for patients with breathing conditions like asthma or COPD — chronic obstructive pulmonary disease. Afrezza is also not recommended for patients who smoke. The FDA decision that Afrezza will not substitute for long-term injectable insulin use was disappointing.
Dr. Jean-Marc Guettier at the Food and Drug Administration’s drug evaluation center stated in a June 27th press release, “Today’s approval broadens the options available for delivering mealtime insulin in the overall management of patients with diabetes who require it to control blood sugar levels.”
These are nice words from the FDA, but the investor expectations for Afrezza were highly inflated, and the warning label will obviously hurt the growth of sales.
The author welcomes feedback and will respond to reader comments.
COMMENTS
Please let us know if you're having issues with commenting.