Nearly two dozen state attorneys general sent a letter to Food and Drug Administration (FDA) Commissioner Dr. Robert Califf on Friday, urging the agency to reverse its “illegal and dangerous” decision to abandon certain restrictions concerning abortion pills.
Twenty-two attorneys general signed on to the letter less than a week after the Biden administration made several moves to make abortion pills more accessible across the country. In early January, the FDA made a regulatory change allowing retail pharmacies to offer mifepristone — the first pill used in a two-drug medication abortion regimen — in-store and by mail order, though patients will still need a prescription from a certified health care provider.
Attorney General Steve Marshall Condemns the FDA’s “Illegal and Dangerous” Decision to Abandon Restrictions on Abortion-Inducing Drugs https://t.co/pHXZh9tdBI pic.twitter.com/QRmAFdS9FL
— Attorney General Steve Marshall (@AGSteveMarshall) January 13, 2023
At the same time, the FDA officially removed the in-person requirement from its regulatory rule book for mifepristone, meaning women will continue to be able to obtain a prescription for the abortion pill via telemedicine. The FDA made the move the same day President Joe Biden’s Department of Justice (DOJ) cleared the U.S. Postal Service to deliver abortion drugs to states with abortion restrictions and bans, offering “limited assurances that a federal law addressing the issue won’t be used to prosecute people criminally over such mailings,” according to Politico.
The attorneys general told Califf that the FDA’s “decision to abandon commonsense restrictions on remotely prescribing and administering abortion-inducing drugs is both illegal and dangerous.”
“In direct contravention of longstanding FDA practice and congressional mandate, the FDA’s rollback of important safety restrictions ignores both women’s health and straightforward federal statutes. We urge you to reverse your decision,” the letter reads. “The authority to regulate abortion lies with the people and their elected representatives. In our states, we prioritize the health and safety of women and children and our laws reflect this … Our States will not yield to the Administration’s radical pro-abortion policies.”
The letter notes that when FDA first approved mifepristone in 2000, “the agency recognized that the drug carried serious risks for women, including infection and bleeding” and historically imposed several restrictions to keep women safe.
“According to the REMS (Risk Evaluation Mitigation Strategy), mifepristone could only be prescribed by a qualified physician and administered in a hospital, clinic, or medical office and only by or under the supervision of such a physician,” the letter details. “Until recently, the FDA adhered to the judgment that these requirements—which prohibited remotely prescribing mifepristone—are necessary to mitigate the serious health risks to women who take the drug.”
“Many states have rightly recognized that drugs like mifepristone are dangerous, especially when prescribed and administered remotely. … It is not seriously disputed that provisions like these are lawful and necessary to protect women’s health,” the letter continues.
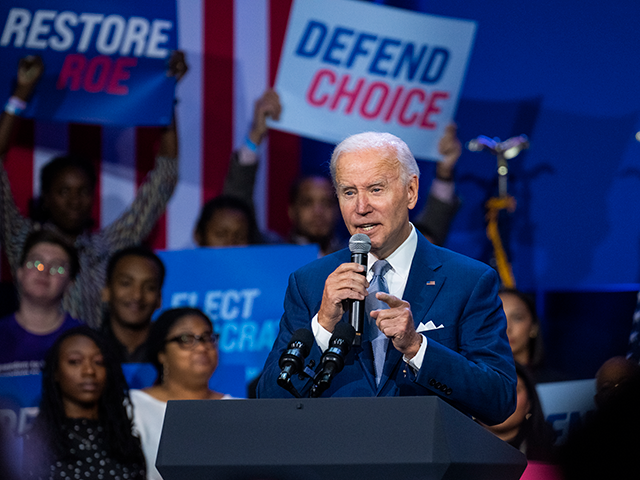
President Joe Biden speaks about the importance of electing Democrats who want to restore abortion rights, during an event hosted by the Democratic National Committee at the Howard Theatre in Washington, D.C., on Tuesday, October 18, 2022. (Tom Williams/CQ-Roll Call, Inc via Getty Images)
The FDA’s decision to allow the remote prescription of mifepristone is not the result of an analysis on how to help promote women’s health, but rather a modification made to “reduce burden on the health care delivery system and to ensure the benefits of the product outweigh the risks,” the letter reads, quoting the FDA’s own website. The attorneys general wrote that the problems with the change in policy “are legion.”
“Most importantly, the FDA has ignored its responsibility to protect health and safety by prioritizing a reckless pro-abortion policy over women’s health. Though there are risks to a woman of using these drugs at any point in pregnancy, abortion-inducing drug are riskiest when used later in pregnancy,” the letter reads. “This means that accurately determining the date of pregnancy is critical for women’s safety. And that determination will be accurate only if made in-person via ultrasound.”
“By permitting and promoting the remote use of abortion drugs, you are endangering the lives of women. Of course, your policy enthusiastically endangers the lives of unborn children who may be even older and more developed than could be known without an in-person examination,” the letter continues.
The attorneys general also called out the Department of Justice for issuing a creative opinion that is “contrary to the plain text of federal law.”
“To be sure, the Biden Justice Department recently tried to invent an exception to the law, opining that the law ‘is narrower than a literal reading might suggest.’ But the statute couldn’t be plainer, and it is no suggestion: a violation is a felony that carries five years’ imprisonment. And yet, you now encourage physicians to facilitate remote abortions and pharmacies to order and provide abortion drugs,” the letter reads.
Attorneys general from Alabama, Alaska, Arkansas, Florida, Georgia, Idaho, Indiana, Iowa, Kentucky, Louisiana, Mississippi, Missouri, Montana, Nebraska, Ohio, South Carolina, South Dakota, Tennessee, Texas, Utah, and West Virginia signed off on the letter and ultimately concluded that the federal government does not have the power to override state laws.
“Though the FDA has abdicated its responsibility to protect women’s health, we have not. To be crystal clear, you have not negated any of our laws that forbid the remote prescription, administration, and use of abortion-inducing drugs. The health and safety of our citizens—women and children included—is of paramount concern. Nothing in the FDA’s recent changes affects how we will protect our people,” they concluded.
COMMENTS
Please let us know if you're having issues with commenting.