Sen. Ron Wyden (D-OR) told the Biden administration that it should ignore an upcoming potential court order that would block the distribution of mifepristone, the first pill used in a two-step medication abortion regimen.
During a speech on the Senate floor Friday, Wyden spoke about a case spearheaded by Alliance Defending Freedom (ADF) on behalf of four national medical associations and several doctors against the U.S. Food and Drug Administration (FDA), Fox News reported on Monday. The lawsuit, which was filed in the U.S. District Court for the North District of Texas, points to six discrete agency actions since the legalization of mifepristone and misoprostol in 2000 and asks the court to hold the actions unlawful, which would ultimately take mifepristone off the market.
“In the coming days a lawless Trump-appointed judge is expected to ban access to abortion medication nationwide,” Wyden wrote on Twitter. “I’m calling on the FDA to protect the safety of every woman in America by keeping the drug on the market no matter the ruling.”
In the coming days a lawless Trump-appointed judge is expected to ban access to abortion medication nationwide. I'm calling on the FDA to protect the safety of every woman in America by keeping the drug on the market no matter the ruling. https://t.co/yWAbAOUTFr
— Ron Wyden (@RonWyden) February 16, 2023
The ADF’s complaint states that the “only way” the FDA could have approved chemical abortion drugs “was to use its accelerated drug approval authority, necessitating that FDA to call pregnancy an ‘illness’ and argue that these dangerous drugs provide a ‘meaningful therapeutic benefit’ over existing treatments.”
“But pregnancy is not an illness, nor do chemical abortion drugs provide a therapeutic benefit over surgical abortion. In asserting these transparently false conclusions, the FDA exceeded its regulatory authority to approve the drugs,” the complaint states.
The lawsuit alleges that the FDA never studied the safety of mifepristone under the labeled conditions of use, ignored the potential impacts of the hormone-blocking regimen on the developing bodies of adolescent girls, disregarded evidence that chemical abortion drugs cause more complications than surgical abortion, and eliminated necessary safeguards for pregnant girls and women who take the regimen.
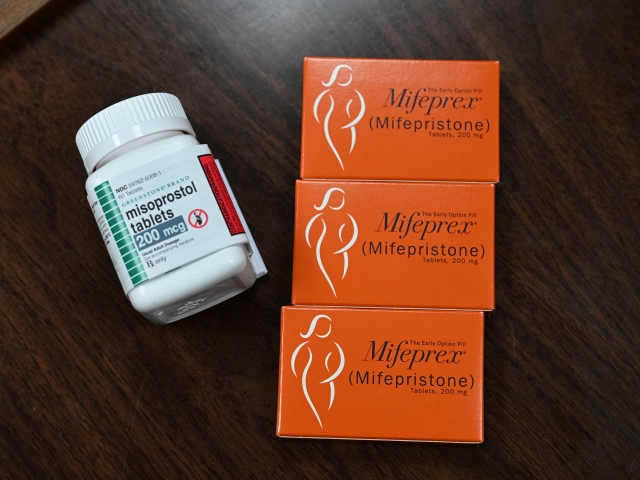
Mifepristone (Mifeprex) and Misoprostol, the two drugs used in a medication abortion, are seen at the Women’s Reproductive Clinic, which provides legal medication abortion services, in Santa Teresa, New Mexico, on June 17, 2022. (Photo by ROBYN BECK/AFP via Getty Images)
The lawsuit further details how, in 2016, the FDA extended the permissible gestational age of the baby for which a girl or woman may take the abortion drugs — from seven weeks gestation to ten weeks gestation. Then in 2019, the FDA allegedly approved a generic version of the drug “without requiring any new clinical investigations or studies that evaluated the drug’s safety and effectiveness under the requirements” under several laws, according to the complaint.
In 2021, the FDA allowed abortionists to send mifepristone through the mail, which the ADF says was “in direct violation of federal law.” The FDA recently made permanent its rule to allow women and girls to receive a prescription for mifepristone via telemedicine. The complaint alleges:
All of the FDA’s actions on chemical abortion drugs—the 2000 approval, the 2016 major changes, the 2019 generic drug approval, and the two 2021 actions to eliminate the in-person dispensing requirement—failed to acknowledge and address the federal laws that prohibit the distribution of chemical abortion drugs by postal mail, express company, or common carrier. Instead, the FDA’s actions permitted and sometimes even encouraged these illegal activities.

COMMENTS
Please let us know if you're having issues with commenting.