Scientists are working on antiviral pills to treat the Chinese coronavirus, a prospect that could come to fruition “within the next several months.”
Researchers are working on oral antiviral drugs to treat the coronavirus as the Biden administration continues to push vaccines and vaccine booster shots for those eligible. Clinical trials of the drugs are underway, and results are expected in the coming months.
“I think that we will have answers as to what these pills are capable of within the next several months,” Carl Dieffenbach, director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, said, according to NBC News.
Antivirals, such as Tamiflu, already exist for other common ailments, generally reducing the severity and longevity of the illness if given in the ideal timeframe.
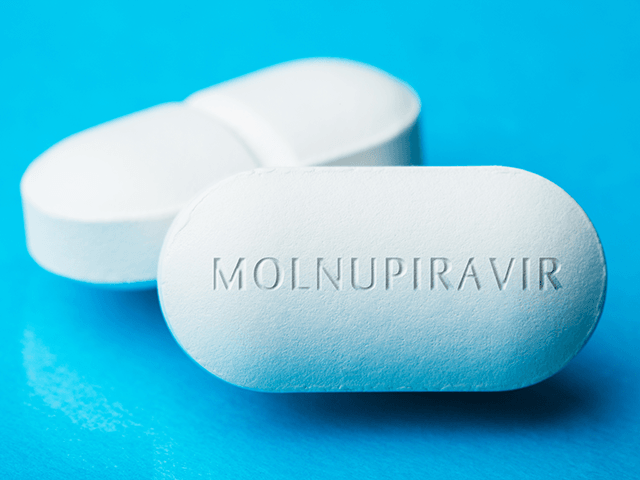
Dieffenbach told NBC News that molnupiravir, from Merck & Co. and Ridgeback Biotherapeutics, is the “top contender” for the antiviral coronavirus pill:
Two others are a candidate from Pfizer, known as PF-07321332, and AT-527, an antiviral produced by Roche and Atea Pharmaceuticals.
They work by interfering with the virus’s ability to replicate in human cells. In the case of molnupiravir, the enzyme that copies the viral genetic material is forced to make so many mistakes that the virus can’t reproduce. That, in turn, reduces a patient’s viral load, shortening infection time and preventing the kind of dangerous immune response that can cause serious illness or death.
…
Clinical trials have followed, including an early trial of 202 participants last spring that showed that molnupiravir rapidly reduced the levels of infectious virus. Merck Chief Executive Robert Davis said this month that the company expects data from its larger phase 3 trials in the coming weeks, with the potential to seek emergency use authorization from the Food and Drug Administration “before year-end.”
Notably, the antiviral medication remdesivir has been approved to treat the Chinese coronavirus, although it is administered through an IV, not in pill form. It is also generally reserved for patients who are hospitalized and experiencing more severe symptoms of the illness.
If clinical trials yield positive results and emergency authorization is obtained, Dieffenbach predicts rollout could “begin quickly,” meaning oral medications could soon be an option for those who have contacted the virus.
The news comes as the Biden administration moves to withhold lifesaving coronavirus treatment from southeastern states such as Florida and Alabama, cutting the deliveries of monoclonal antibodies in the name of “equitable distribution.” The Biden administration’s decision followed the president’s divisive coronavirus speech this month, in which he vowed to get certain governors, who disagree with his general pandemic approach, “out of the way.”
Notably, Gov. Ron DeSantis (R) found a workaround and secured additional monoclonal antibody treatments — 3,000 of GlaxoSmithKline’s sutrovimab — for the Sunshine State.
“It’s clearly saving lives. It’s clearly keeping people out of the hospital. But it’s also something that, even short of being hospitalized, this is something that can really knock you on your rump for a week or two … it assists in the recovery,” DeSantis said this week.
“People get better much quicker if they get this treatment,” he added.
COMMENTS
Please let us know if you're having issues with commenting.