Scientists Win Nobel Medicine Prize for Discovery Leading to mRNA Vaccines
Katalin Karikó and Drew Weissman, scientists credited with discoveries leading to the development of mRNA vaccines, received the Nobel Prize.

Katalin Karikó and Drew Weissman, scientists credited with discoveries leading to the development of mRNA vaccines, received the Nobel Prize.

An updated selection of coronavirus vaccines has been approved by health officials and should be available from Wednesday onwards for all Americans aged six months and upwards.
Israel will declare the coronavirus pandemic officially over next month, with the viral disease being downgraded to the status of the flu.
The Biden administration’s long-predicted coronavirus vaccine program tailored exclusively for infants, toddlers, and preschoolers began Saturday on the back of requests from the pharmaceutical industry.

Pfizer announced Wednesday it has completed an emergency submission to the U.S. Food and Drug Administration (FDA) to administer its three-dose coronavirus vaccine to children under the age of 5.
Around five million children between the ages of five and 11-years old in England will be eligible to take a “low-dose” coronavirus vaccine despite the government acknowledging a low risk profile for youngsters dying from the Chinese virus.
Biotech company Moderna announced Wednesday it is approaching the Food and Drug Administration (FDA) for emergency use authorization to enable coronavirus vaccinations to be administered to very young children, those aged between six months and six years of age included.

An expert panel advising the Israeli Health Ministry on the coronavirus recommended administer a fourth dose of the Pfizer/BioNTech vaccine to Israelis aged 18 and above, provided that five months have passed since they received a third shot or recovered from the disease.
The recent death of a 26-year-old New Zealand man from myocarditis was “probably due to vaccination” with a Covid-19 vaccine produced by Pfizer, New Zealand’s Covid-19 Vaccine Independent Safety Monitoring Board (CVISMB) said.
Israeli ministers on Monday voted to ban travel to the United States, Canada and eight other “red” countries amid the spread of the omicron variant.

The sudden emergence of the Omicron variant – and the fear it has generated – has been cited by the White House as a key driver behind President Joe Biden’s new campaign calling on all Americans to get coronavirus booster shots. Seniors will be particularly targeted under the enhanced program.

The World Health Organization (W.H.O.) on Wednesday okayed offering children coronavirus jabs as approved for individual age groups, adding it should be done alongside the global priority of sharing of vaccines for all.

Amnesty International accused Pfizer, a U.S.-based multinational pharmaceutical giant, on Thursday of using “misleading language” to suggest it has supplied poor countries with greater supplies of coronavirus vaccines than it actually has.
Fictional Sesame Street muppets have taken to social media to encourage children to get vaccinated against the coronavirus.

Chicago Public Schools (CPS) announced it will be canceling school on November 12 for “Vaccination Awareness Day.”

San Francisco will require that children ages five through eleven provide proof of vaccination to gain access to indoor businesses.
The Food and Drug Administration (FDA) has now approved the Moderna and Johnson & Johnson booster shots for the vulnerable.
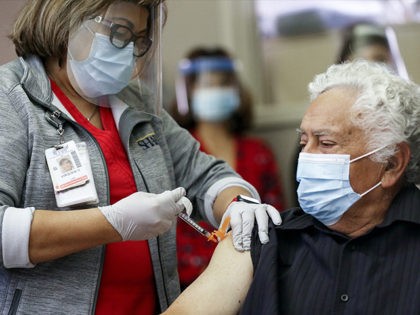
Singapore, which has a Chinese coronavirus vaccination rate of 82 percent, recorded its highest single-day increase in new locally transmitted cases of the virus Tuesday with 3,994 infections.

The advent of coronavirus booster shots could rake in billions of dollars in profits for companies like Pfizer and Moderna, according to the Associated Press.
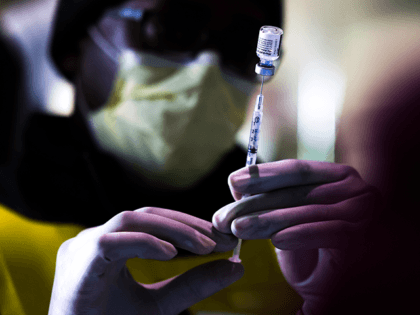
Biden Education Secretary Miguel Cardona said Thursday he not only supports mandatory Chinese coronavirus vaccines for eligible schoolchildren, he expects states to “make sure it happens.”

Kaiser Chiefs lead singer Ricky Wilson has been criticised for encouraging fans to cheer for their vaccine brands, with people responding in rapturous cries and arm-waving described as creepy and cult-like.

Pfizer and BioNTech are set to seek FDA approval in November for their COVID vaccine to use in children between 6 months and 5 years old.

Producers of the Pfizer vaccine, BioNTech, are preparing to request clearance to vaccinate children five and older, and the company is ready to begin making smaller doses for children younger than 12.
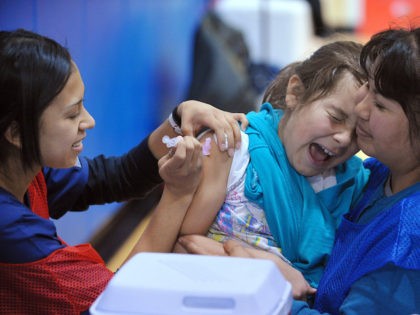
Israel will likely achieve herd immunity for coronavirus in the next month or two, top health officials said as the Delta variant continues to rage through the country.
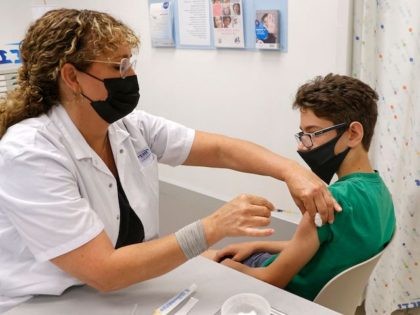
Israel is leading the world in coronavirus cases per capita over the past seven days, a study published on Tuesday by Oxford University shows.

The British government says it is preparing to vaccinate children ages 12-15 against the coronavirus, even though the inoculation campaign has not yet been approved by the country’s vaccine advisors.

Defense Secretary Lloyd Austin on Wednesday issued a memorandum directing the military services to begin full vaccination of all service members following FDA approval of the Pfizer-BioNTech coronavirus vaccine on Monday.
Israel opened the third booster dose of the Pfizer vaccine to over 30s on Tuesday, as the country came close to an all-time high with close to 10,000 reported daily coronavirus infections.
Scientists said the data is not “compelling enough to recommend third shots to most of the American population right now.”

Sixteen and 17-year-olds will be offered vaccination against the Chinese coronavirus without their parents’ consent, government scientists have said.

The British government will reportedly begin rolling out 32 million “booster” vaccines by next month to all those over the age of 50.

TARTU, Estonia (AP) – With her father in tow, 13-year-old Gloria Raudjarv marched through a vaccination centre inside a sports hall in Estonia’s second-largest city and up to a nurse for her first dose of the COVID-19 vaccine.
The Pfizer-BioNTech coronavirus vaccine’s efficacy dropped from 96 percent to 84 percent over six months, according to data published Wednesday.
Three countries have reached out to Israel to obtain one million vaccines rejected by the Palestinians, the Haaretz daily reported Sunday.
The Palestinian Authority called off an agreement under which Israel would transfer one million doses of coronavirus vaccines to it, citing the close expiration date of the Pfizer vials, despite the Israel Health Ministry’s insistence the vaccines were “perfectly sound” and identical to those administered to Israelis.
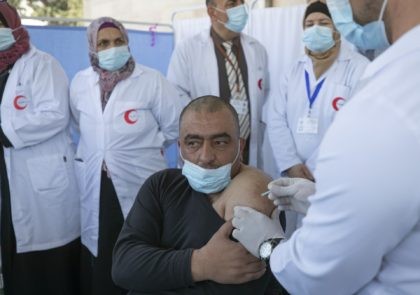
Israel announced the dispatch of around one million-plus coronavirus vaccines Friday to help neighboring Palestinians through their own stumbling vaccination rollout.
Dr. Anthony Fauci has not answered under oath when he knew the Pfizer vaccine received phase 3 approval and if the approval was hidden until after the presidential election.

The United Kingdom has given temporary approval to the Pfizer jab for 12-15 year olds after the govt “carefully reviewed” data for the age group.

Europe can achieve herd immunity against the coronavirus within the next four months, the head of pharmaceutical company BioNTech said.

Pfizer say they will provide 100 million more doses of their coronavirus vaccine to the European Union this year.
