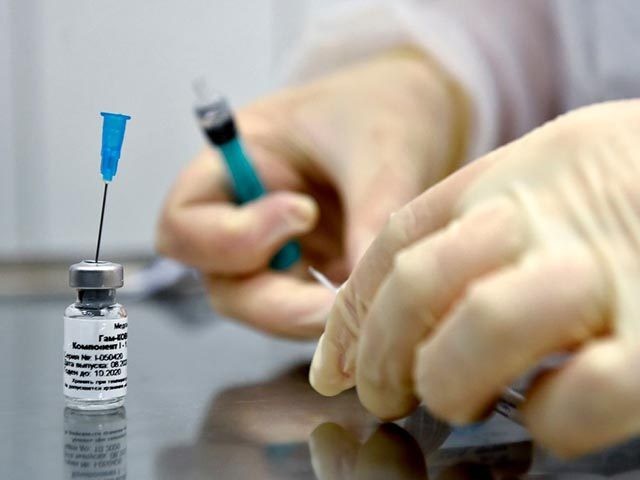
Venezuelan President Nicolás Maduro announced Wednesday that his socialist regime has chosen volunteers to participate in clinical trials for the Russian coronavirus vaccine candidate Sputnik V, according to Russian outlet Tass.
Venezuela received a shipment of Sputnik V earlier in October, Reuters reported. The clinical trials will include 2,000 volunteers, Health Minister Carlos Alvarado said at the time.
“The Russian vaccine is already in Venezuela; the trials are already starting; there are 2,000 volunteers,” Maduro said on Wednesday.
The impoverished South American nation, currently struggling under severe economic downturn from socialist mismanagement of the country, has suffered a severe outbreak of the coronavirus, which has stricken officials at the highest levels of the regime.
The World Health Organization has praised the Maduro regime for its response to the pandemic and its cooperation with the international community.
Authorities have implemented harsh measures to curb the spread, sending anyone showing the slightest symptoms to hospitals ill-equipped to handle the inflow of patients.
Russian President Vladimir Putin announced in August that he had approved Sputnik V for mass distribution despite it not having cleared all phases of clinical trials; he further noted that his daughter had received the vaccine but did not specify which daughter.
Kremlin spokesperson Dimitry Peskov said in late September that Putin planned to get vaccinated against coronavirus to enable a diplomatic trip to South Korea. Though Peskov did not state what vaccine Putin would use, he implied the Russian president would opt for the Russian-developed candidate.
Despite a lukewarm reception from the international community — which was largely skeptical of the Russian vaccine’s safety and effectiveness — several Russian allies, including Venezuela, enthusiastically embraced it, opting to receive large shipments for clinical trials.
Philippine President Rodrigo Duterte personally volunteered to take the vaccine in early August, though his age and health conditions do not allow him to do so.
“When the vaccine arrives, I will have myself injected in public. Experiment on me first, that’s fine with me,” he said.
Belarusian President Alexander Lukashenko claimed the Kremlin promised Belarus would be the first nation to receive an effective Russian vaccine. Lukashenko expressed confidence in the vaccine’s safety, citing Putin’s willingness to test it on his own family. “I believe him for a reason: he tested this vaccine on his family,” he said.
Some countries have taken a more measured approach to Sputnik V, remaining open to its potential but demanding more data before allowing widespread distribution. India’s Central Drugs Standard Control Organisation, which regulates pharmaceuticals, rejected a proposal for large-scale clinical trials, instead advising a smaller, localized trial-run to gather more data.
Sputnik V began phase three trials in Russia on September 1 with 40,000 participants, according to the Hindustan Times.
COMMENTS
Please let us know if you're having issues with commenting.