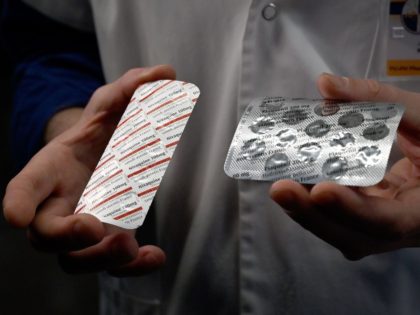
DeSantis: Biden Administration Hampering Efforts to Get Floridians Treatment for Coronavirus
GOP Florida Gov. Ron DeSantis said the Biden administration is hostile toward his efforts to get coronavirus treatment for Floridians.

GOP Florida Gov. Ron DeSantis said the Biden administration is hostile toward his efforts to get coronavirus treatment for Floridians.

Drugmaker Merck has asked United States regulators to authorize its pill for treating the Chinese coronavirus, the Associated Press (AP) reported Monday.

A “family planning fellow” at the University of Michigan has created a series of videos showing health care workers how to interact with biological men and women who are living as their opposite sex. “Transgender and gender diverse people face
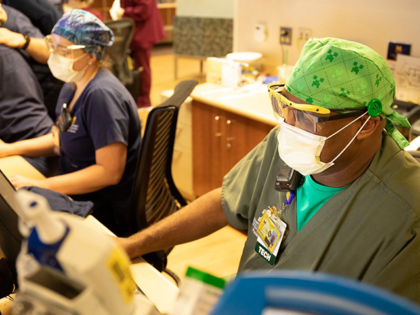
The Food and Drug Administration (FDA) approved Eli Lilly’s coronavirus antibody treatment for emergency use.
A study by researchers at Harvard University’s School of Public Health found social distancing practices will likely be required until 2022 unless treatment or a vaccine becomes available to counter the spread of the infection caused by the novel coronavirus.

Food and Drug Administration (FDA) administrator Dr. Stephen Hahn told Breitbart News exclusively on Wednesday that his agency is working with private industry leaders to bring more antibody testing and promising potential plasma-based treatments for the coronavirus to the American public.

The world economy is collapsing because of the terror and mounting death toll caused by the Coronavirus pandemic. But the anti-malarial drug chloroquine is effective both as a prophylactic and treatment for the virus – and the medical establishment has known about this since at least the SARS coronavirus outbreak in 2005. What the hell is going on?