Disney-Themed Hand Sanitizer Products Recalled over Cancer Link
Two hand sanitizer products with Disney characters on the bottles have been recalled because some ingredients have been linked to cancer and other serious medical problems.

Two hand sanitizer products with Disney characters on the bottles have been recalled because some ingredients have been linked to cancer and other serious medical problems.

The Food and Drug Administration (FDA) on Tuesday authorized Pfizer-BioNTech’s and Moderna’s second booster shots for the Chinese coronavirus for those 50 and older and “certain” immunocompromised individuals.
Biotech company Moderna announced Wednesday it is approaching the Food and Drug Administration (FDA) for emergency use authorization to enable coronavirus vaccinations to be administered to very young children, those aged between six months and six years of age included.

Pfizer and partner BioNTech approached U.S. regulators Tuesday renewing their call for authorization to administer a fourth coronavirus shot as a booster for seniors.
The FDA recently discovered a rodent infestation at a Family Dollar distribution facility which led to the temporary closure of 404 stores.

An executive director at the U.S. Food and Drug Administration (FDA) believes that coronavirus vaccines have “not been as effective as they’re expecting,” according to a video released by Project Veritas.
Four Senate Republicans bailed out Senate Majority Leader Chuck Schumer (D-NY) on Monday by voting to advance the nomination of Dr. Robert Califf as the head of the Food and Drug Administration.

Pfizer and BioNTech are delaying their Food and Drug Administration (FDA) request for the authorization of their Chinese coronavirus vaccine for children under the age of five, the company announced on Friday.
“Wasteful” NIH-funded animal experiments have come under fire for alleged violations of federal spending transparency law.

A federal judge gave the FDA eight months to release data pertaining to the safety of Pfizer’s coronavirus vaccine after the FDA had asked for 75 years.
President Joe Biden sounded the alarm about the rise in coronavirus cases on Tuesday, but made no mention of existing or future treatments for the virus.

The Food and Drug Administration (FDA) eased restrictions Thursday on at-home drug-induced abortions by allowing women and girls to end their pregnancies via mail-order pills without having to see an abortion provider in person.

The Biden administration is unlikely to see its long-delayed Food and Drug Administration (FDA) director confirmed before the end of the year, despite fears of a new coronavirus variant and a winter surge, thanks to an avoidable paperwork blunder.

The FDA asked a judge this week if the agency could have 55 years to fully release the data used to license Pfizer’s coronavirus vaccine.
Sen. Joe Manchin (D-WV) on Friday came out against President Joe Biden’s nominee for Food and Drug Administration (FDA) commissioner, Dr. Robert Califf, citing his poor handling of the opioid epidemic.

Ellume has recalled its at-home coronavirus test due to potential false positive results, according to the U.S. Food and Drug Administration (FDA).

The U.S. Food and Drug Administration (FDA) is expected to meet Tuesday and consider a proposal to extend coronavirus vaccinations making 28 million younger children down to age five eligible for the shots in November.

The Food and Drug Administration (FDA) has now approved the Moderna and Johnson & Johnson booster shots for the vulnerable.
Joe Biden specifically condemned reports that claimed Southwest Airlines employees protested the mandates last weekend which led to major service delays.

The Food and Drug Administration (FDA) is questioning the evidence provided by Johnson & Johnson in its application for authorization of its coronavirus booster shot, according to a document released prior to the October 15 meeting with FDA vaccine advisers.

Pfizer announced Thursday it applied to the Food and Drug Administration (FDA) for approval of its vaccine for 5-11-year-olds — a significant move, as no coronavirus vaccine has been approved for children younger than 12.
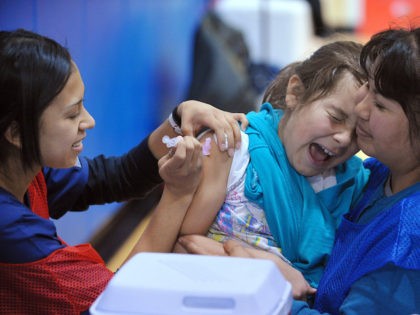
A plurality of voters, as well as a majority of Republicans and independents, are “not confident” coronavirus vaccines are “necessary and appropriate” for children, a Convention of States Action/Trafalgar Group survey released Tuesday found.
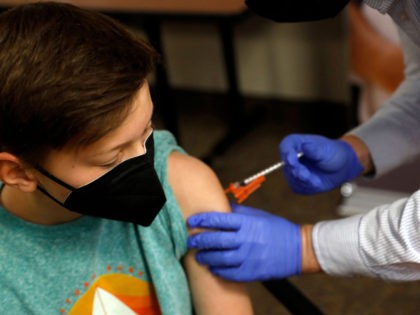
Biden Education Secretary Miguel Cardona said Thursday he not only supports mandatory Chinese coronavirus vaccines for eligible schoolchildren, he expects states to “make sure it happens.”

A meeting in March with Dr. Anthony Fauci and Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky raises questions of “outside influence” regarding President Joe Biden’s federal vaccine mandate, Breitbart News has learned exclusively.

The FDA approved Pfizer coronavirus booster shots on Wednesday for senior citizens and those at high risk for coronavirus.
After a dismal week for the Biden administration on several fronts, the New York Times published a “puff piece” profile on White House press secretary Jen Psaki, which repeatedly praised her as “straightforward” and “professional,” Fox News reported Sunday.

Scott Gottlieb, the former commissioner of the Food and Drug Administration (FDA), admitted during an interview on Face the Nation that the six foot social distancing rule recommended by public health officials for months on end was actually “arbitrary in and of itself,” and he noted that “nobody knows where it came from.”

The Biden-Harris administration Friday suffered three crises, which includes droned Afghan civilians, unapproved of coronavirus booster, and the recalled French ambassador from the United States.

On Friday’s broadcast of CNN’s “Situation Room,” FDA Vaccines and Related Biological Products Advisory Committee member Dr. Paul Offit stated that “in many ways, the FDA and CDC were marginalized” by the Biden administration’s handling of coronavirus vaccine boosters, but

Bad news for President Joe Biden piled up right after he left the White House early on Friday for a long weekend at the beach.

A Food and Drug Administration (FDA) panel voted Friday afternoon to reject a plan to provide Pfizer-BioNTech coronavirus booster vaccines for most Americans due to a lack of data showing the additional doses are safe and effective.
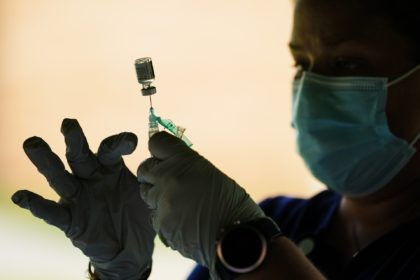
Pfizer and BioNTech are set to seek FDA approval in November for their COVID vaccine to use in children between 6 months and 5 years old.

Pfizer prefaced its upcoming presentation to the Food and Drug Administration (FDA) to discuss its application for Chinese coronavirus booster shots by asserting data shows vaccine efficacy wanes over time.

Two senior vaccine regulators, who are departing the Food and Drug Administration (FDA), published a paper blasting coronavirus booster shots, something discussed and embraced by the Biden administration and federal health officials.
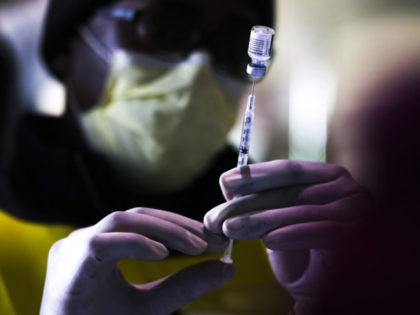
Producers of the Pfizer vaccine, BioNTech, are preparing to request clearance to vaccinate children five and older, and the company is ready to begin making smaller doses for children younger than 12.

The Associated Press recently issued a correction to a report alleging that 70% of poison control cases in Mississippi were linked to the drug ivermectin.
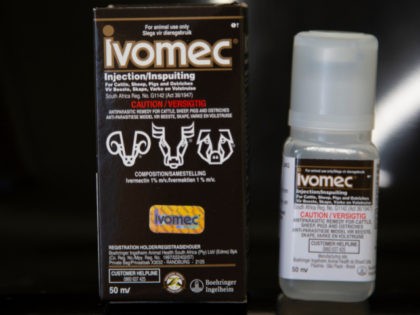
Throughout his presidency, Joe Biden has constantly repeated the mantra that he would “follow the science” on coronavirus policy, but two Food and Drug Administration (FDA) officials have just resigned in protest of what they claim is the Biden administration perpetuating an atmosphere of pressure and intimidation to push the coronavirus booster shots.

A pair of top Food and Drug Administration (FDA) officials overseeing the review of COVID-19 vaccine applications will depart the agency this coming fall, according to a Tuesday report.
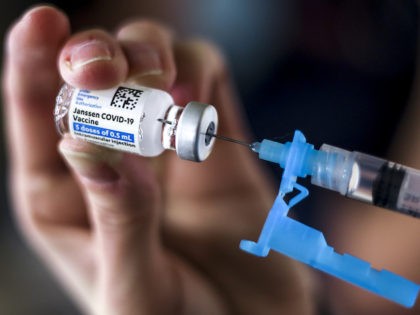
Infectious disease specialist Anthony Fauci is now agreeing with GOP FL. Gov. Ron DeSantis that antibody treatments can fight coronavirus.

U.S. regulators are likely to greenlight a coronavirus booster vaccine for vaccinated adults beginning at the six month gap following the previous shot instead of eight-months, according to the Wall Street Journal.