It’s always a challenge to notice something that isn’t there, but should be there—the absence of a presence, one might say. And so, as we read the article in the April 28 Washington Post, “There’s a New Sheriff in town in Silicon Valley—the FDA,” we can hunt for what, or who, is missing.
Indeed, as we peruse the Post article, detailing the feud between young California techsters and the venerable DC-based Food and Drug Administration, we can think back to Silver Blaze, the 1892 story by Arthur Conan Doyle, in which Sherlock Holmes solves the case by observing that the guard dog did not bark when it should have.
If Holmes were still sleuthing today, he might deduce that what’s most interesting about the Post story is the glaring absence of elected officials involved in a life-and-death scientific struggle over medical testing—a struggle in which billions of dollars, too, are at risk. Indeed, this situation might be called The Curious Case of the Missing Political Leadership on Health Technology.
This case has been growing curiouser and curiouser for a while. Back in December, The Wall Street Journal’s Thomas M. Burton reported on the medical-testing issue as a kind of regulatory shootout; his headline asked, “Is Lab Testing the ‘Wild West’ of Medicine?”
As the Journal noted, perhaps because the FDA hasn’t worried much about the testing sector, some 100,000 different medical tests have been developed. Is that too many? Or too few? Or are they not of the right kind? It all depends on whom you ask. As lab-test advocates warned in the Journal article, too much regulation could “stifle innovation.”
This is no abstract theoretical question; the human stakes are, indeed, profound: In 2013, for example, the actress Angelina Jolie was one of many woman who have elected to undergo a pre-emptive double mastectomy after a medical test told her that she had the BRCA gene, which can lead to cancer. Yet such medical decisions continue to be controversial: Dr. Otis Brawley, medical director of the American Cancer Society, told the Journal that, in his opinion, BRCA lab results are of “unknown significance.”
Yes, it is, indeed, a painful situation, in that Jolie, who presumably had access to the best cancer doctors, came to a conclusion at odds with the chief doctor at the American Cancer Society. And this unreconciled dilemma is a reminder that for many medical questions, the science is still unresolved. Researchers may ultimately arrive at a One Best Way, or perhaps they will conclude that there is, in fact, no one-size-fits-all answer.
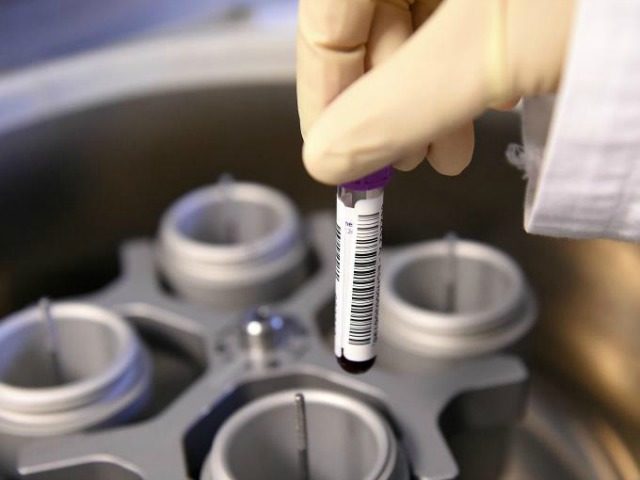
The key issue within the medical-testing issue is the “liquid biopsy.” That’s a test that can, in theory at least, ascertain from a single drop of blood everything that’s wrong with a patient. Such a test could be called the holy grail of medical technology; indeed, one of the companies in the liquid-biopsy sector is called just that—Grail.
As the Post observes, progress against cancer, just to name one malady, is urgently needed, and that starts with finding it:
Being tested for cancer today often means having a slice of tissue cut out—which can be painful and dangerous—and waiting days or even weeks for the results to come back. The promise of liquid biopsies is that the same information might be available based on an extremely low-risk blood draw that takes mere minutes.
Reporter Ariana Eunjung Cha continues:
The [liquid biopsy] concept is so simple and potentially inexpensive that it could upend practically everything about the disease. Healthy people would be able to walk into their doctor’s office for an annual checkup and know whether they had cancer well before it becomes life-threatening. Doctors would be able to track their patients’ responses to therapies almost in real time by studying which cancerous mutations are in the mix and in what concentration.
Yet of course, to say that liquid biopsies would be revolutionary if they worked is not the same as proving that they do, in fact, work. Indeed, the Post piece introduced a note of skepticism: “Some industry observers [are] worried that pseudoscience is being confused with innovation.”
Yet whether or not liquid biopsies are pseudoscience or good science, some of the smartest people in America are believers, and they are putting their own money, and lots of it, behind their beliefs. As the Post noted, in 2015 alone, investors, mostly in and around Silicon Valley, have pumped more than $10 billion into the medical-testing sector—and one of the investors is Jeff Bezos, owner of the Post. The result, the newspaper added, has been “a tidal wave of new health-related gadgets, apps and tests,” mostly aimed at that holy grail of single-drop testing.
Yet in response, the FDA, which has previously been mostly hands-off on medical testing, has decided to start regulating, in a big way. The agency has deemed that a number of testing firms, including Theranos, 23andMe, Lumosity and Pathway Genomics, are in violation of one regulation or another—and socked them with warning letters and fines.
Of these companies, perhaps the best known, or most notorious, is Theranos, which has found itself in real trouble with Uncle Sam. Breitbart’s Chriss W. Street has written a string of articles on Theranos, none of them laudatory.
So who’s right? Should we have faith in scientific visionaries and their investors? Or in regulators like the FDA? It’s hard for a layperson to know for sure.
But we do know three things. First, risk is part of the scientific process. Second, Uncle Sam has, at best, an ambivalent relationship with innovation, especially medical innovation. And third, the Obamans have been particularly cool to medical testing because of the administration’s oft-stated desire to “bend the cost curve” of health care—that is, get spending down.
Let’s look at each point in turn.
First, as to risk-taking, we know that the pioneers in any sector take risks. In aviation history, for example, most of the early test pilots were killed in accidents. And in health care, the doctrine of informed consent—that is, you know what you’re getting yourself into—has enabled volunteers to take risks for the sake of medical science, as well as for themselves. Not every experiment has worked out happily, of course, but overall, medicine has advanced.
Second, as to the federal government, it is, by its nature, a bureaucratically cautious beast. Long ago, bureaucrats discovered that, for them, taking action, any action, entails more downside than upside. That is, a civil servant has more to lose by getting something wrong than to gain by getting something right. So the result, quite predictably, is that regulatory bureaucrats tend to regulate in a risk-averse fashion—they would rather say “no” than “yes.” And that’s why the FDA actually approved fewer drugs in 2015 than it had twenty years earlier, in 1996.
As they say, today’s FDA wouldn’t approve aspirin—too risky.
Indeed, hanging over all FDA issues, including the liquid biopsy sector, is the specter of “Eroom’s Law”—that’s Moore’s Law spelled backward. Whereas Moore’s Law describes the rapid decrease in the cost of computing, Eroom’s Law describes the rapid increase in the cost of developing a new medical drug, mostly because of greater regulatory hurdles.
Third, the Obama administration, in attempts to keep the cost of Obamacare under control, has long been communicating its reluctance on the subject of testing to the rest of the federal government, including the career staff. Why? Because medical testing encourages medical treatment, and medical treatment costs money.
In 2009, the Obama-ized Department of Health and Human Services, led by then-Secretary Kathleen Sebelius, prevailed on the Preventive Services Task Force, an HHS sub-unit, to issue revised recommendations on mammograms, calling upon women to wait to age 50 before they begin such testing. Earlier, the recommended age had been 40.
The suspicion arose immediately that the feds wanted women to wait because waiting would save the system money—which is not the same, of course, as saving lives. The Obamans denied any such intention, even as they backed down, sort of. And ever since, the mammogram issue has been a tangle of conflicting recommendations. A Popular Science headline captured the confusion: “The Confounding Commandments of Cancer Screening.”
But our particular subject here is The Curious Case of the Missing Political Leadership on Health Technology. And so as we work our way through both the Journal and Post stories on medical testing, we have our “eureka” moment when we realize what’s missing. And that is, not a single elected official is mentioned by name in either piece. (Although the Journal noted in passing, “The House Energy and Commerce Committee has circulated draft legislation that would sharply reduce the FDA’s authority over medical testing.” That’s a reference to the pro-science diligence of Rep. Fred Upton (R-Mich.), the hard-working chairman of the committee. Upton is known to be skeptical of the FDA’s analytical sapience and, therefore, doesn’t want to see its regulatory reach extended; indeed, Upton has emerged as perhaps the most dogged—if mostly unheralded—congressional champion of medical progress.)
Yet aside from Upton, the absence of a political presence in this FDA-vs.-medical-test companies is a noticeable pattern—and it is not an accident. Most elected officials prefer to be on the sidelines, free from any responsibility.
After all, medical research is risky. People get hurt and even die. Everyone in the field remembers thalidomide, back in the 50s and early 60s, and, more recently, there was the case of Jesse Gelsinger, the teenager who died in a clinical trial run by the University of Pennsylvania, as the school was exploring gene therapy. Gelsinger’s death was a tragedy, but let’s be blunt: His condition was tragic even before the therapy, as he suffered from a rare malady, Ornithine Transcarbamylase Deficiency, a genetic disease of the liver that was killing him. Still, Gelsinger’s death paralyzed gene therapy for years.
So naturally, politicians—most of whom, in their way, are as risk averse as bureaucrats, and for the same reasons—wish to stay far away from FDA issues.
Again, with the exception of Upton, most elected officials would simply rather not take a position. Indeed, if they can, they will maneuver to get what they really want—that is, deniability.
So if things go great, great—there’s always time to issue a press release praising the good news. But if things go not so great, if somebody dies, well, the pols can’t be blamed because they weren’t involved in the approval process; it was those medical-testing companies, or the FDA, or both, or something.
Yet what’s perhaps most interesting is that the investors who put up that $10 billion in 2015 money have let the politicians—many of whom have counted on the investors for campaign contributions—to get away with this non-stance stance. That is, investors have put up all that money, and yet they get no help from the politicians they helped; they are on their own in their struggle with the FDA.
Yes, reading the Journal and the Post, one sees no visible impact of, for example, California Senators Barbara Boxer and Dianne Feinstein, nor any member of the Golden State’s House delegation—even though Silicon Valley is their home team. It’s possible, of course, that some of them are playing an extensive role behind the scenes, but if they were, such efforts would likely become a public matter. Again, we can safely conclude that these elected officials just aren’t involved.
Nor, interestingly, do we see California Governor Jerry Brown or any local official making a cause for the advancement of medical testing—a cause they might encourage for reasons either of personal sympathy or of their state’s economic development. There is, as we have seen, big money in medicine.
In other words, in the battle between the FDA and the medical-testers, the political class is AWOL—and that’s the way the pols want it.
Of course, the medical testers are hardly without resources, and so they are hardly alone. They have a small trade association, the American Clinical Laboratory Association (ACLA), although it is limited in scope; as of this writing, its Twitter feed consists of just 790 followers. Still, ACLA managed to hire two blue-chip lawyers, Laurence Tribe of Harvard Law School and Paul Clement, a former solicitor general now in private practice, to plead its case to the FDA.
So maybe things will work out for the medical testers and their holy grail of an idea. Maybe all that money, and all that brain-power, will drive the train of medical innovation for the benefit of all of us. It’s worked before.
But maybe not this time. After all, history tells us that in the absence of political leadership, even good science can be waylaid by bad outcomes. This could mean, for instance, a proliferation of lawsuits, or an attack of Naderism, or of Sanders-ism. Or the Greens, who oppose anything that’s good for humans, could get really involved. Or perhaps China gets there first and does to U.S. medical testing what it has done to U.S. manufacturing.
Yes, a negative domestic political climate can mostly shut down an industry, as we have seen with the nuclear industry, as well as coal and, maybe soon, oil.
So what could the medical-testing industry—and, by extension, the forces of all medical progress—do to advance their cause? How could they help themselves, and the rest of us, who might need their stuff to live?
To borrow the old Cold War concept, it’s all about the correlation of forces. That is, who has the political throw-weight. Today, the opponents of medical progress—that is, the aforementioned Naderite-trial-lawyer left, boosted by the Obamanik cost-controllers—must be rated as very strong. So while it might not be fair to label the FDA, overall, as an opponent of medical progress, it is more than fair to say that the Naderites, et al., along with a scandal-hungry MSM, will have the FDA’s back in any policy fight.
And what coherent bloc is strong on the other side? Well, none. As we have seen, there’s ACLA, but it’s small, not strong. And there’s all those Silicon Valley investors; they are, of course, formidably strong, but so far, at least, they haven’t organized themselves into a real political SWAT team to advance medical progress.
Yes, they put in that $10 billion in medical investment, just in a single year, but who knows—maybe that sum, princely as it is to most of us, is too small for SV-ers really to worry too much about. Maybe the Valley figures that making nice with the Obama administration on tech and encryption policy—or on Green goals—is more important than protecting their medical-testing investment. Or maybe, for all their brilliance, the techsters are simply still naive about Washington.
So in the meantime, the FDA regulators are winning—and therefore, some say, squelching. And yes, history and common sense tell us that if the FDA wins in its battle against medical innovation, then the rest of us, as patients, will lose.
But that’s not the FDA’s problem.
COMMENTS
Please let us know if you're having issues with commenting.