Solata Foods Recalls Spinach Products Due to Possible Listeria Contamination
A New York food company is recalling its spinach products due to possible listeria contamination that could sicken consumers.

A New York food company is recalling its spinach products due to possible listeria contamination that could sicken consumers.

The FDA took more than a year to act on a whistleblower report about an Abbott Nutrition factory that was ground zero for a nationwide infant formula shortage.

Thousands of women in states that have abortion restrictions were able to obtain abortion pills last year from providers in blue states.

The FDA is reportedly planning to end its broad ban against anonymous sperm donations from gay and bisexual men.

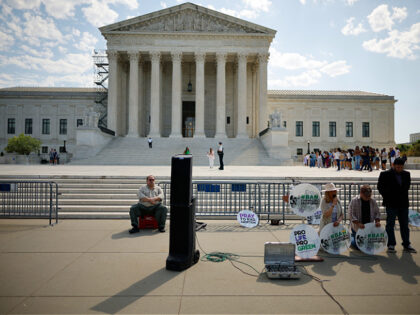
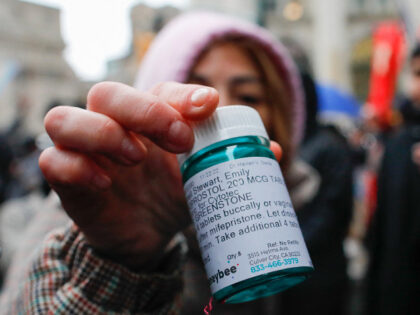
The Supreme Court heard oral arguments in a case that could have a significant impact on how mifepristone is prescribed in the United States.

The Supreme Court is set to hear oral arguments on Tuesday, March 26, in a case that could have a significant impact on how mifepristone — the first drug used in a two-drug medication abortion regimen — is used and prescribed in the United States.
Health officials warned consumers that ground cinnamon sold at stores such as Dollar Tree and Family Dollar were contaminated with lead.

The first over-the-counter birth control pill approved in the U.S. is headed to stores in the coming weeks and will also be available online.

CVS and Walgreens are set to begin selling abortion drugs “within a week,” ahead of a Supreme Court battle challenging the U.S. Food and Drug Administration’s (FDA) rollback of safety restrictions for mifepristone, the first drug used in a two-drug medication abortion regimen.

Elon Musk claims Neuralink’s first human patient with the company’s creepy brain chip implant can now move a computer mouse cursor using just their thoughts.

The Supreme Court set a date for oral arguments in a case about the FDA rolling back safety restrictions for mifepristone.

Interestingly, the study found that the non-pregnant women who requested abortion pills the most were white and over 30 with no children.
More than 675,000 cans of specialty baby formula are being recalled due to possible contamination with a bacteria which can cause serious health risks.

Federal officials say the Coca-Cola Company is recalling approximately 2,000 cases of beverages due to possible “foreign material.”

There has been a spike of illnesses possibly linked to lead in cinnamon applesauce products after the foods were recalled, and federal officials are investigating the matter.

The Supreme Court decided to hear a case about the FDA rolling back safety restrictions around the abortion pill.

The U.S. Food and Drug Administration (FDA) may soon green-light a drug that could help large-breed dogs live longer.

At least 99 infections and two deaths have been linked to a salmonella outbreak stemming from recalled cantaloupes, the Centers for Disease Control and Prevention (CDC) announced Friday.

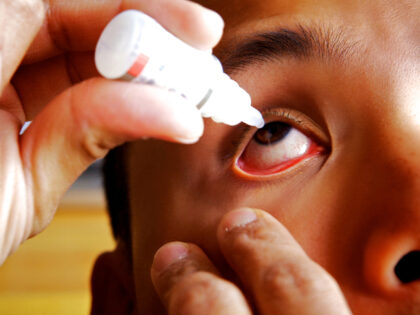
Multiple eye drops have been recalled after a report divulged “insanitary conditions” at a factory in Mumbai, India.
Thousands of people have reportedly shown interest in participating in the FDA-approved human trials of the brain-computer interface developed by Elon Musk’s Neuralink. Test participants will have a computer chip installed in their brain, and can only hope for better results than Musk’s test monkeys, many of which suffered a gruesome fate.

Federal officials on Thursday proposed banning the use of a certain vegetable oil in drinks due to safety concerns.

The FDA is advising parents to throw out WanaBana Apple Cinnamon Fruit Puree Pouches due to “extremely high concentrations of lead” discovered in the product.

A recent investigation has unveiled a harrowing incident involving the results of Elon Musk’s Neuralink brain implant trial on macaque monkeys, sparking a debate about if the technology is ready for human trials. In one gruesome incident, the implant caused a monkeys brain to “rupture,” leading to its untimely death.

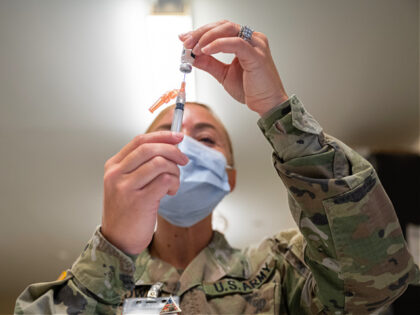
Only 43 out of more than 8,000 service members who were discharged from the military over the Biden administration’s COVID-19 vaccine mandate have sought to rejoin, according to a recent report by CNN.
Elon Musk’s brain-computer interface startup, Neuralink, is facing fresh controversy as it secures approval for human clinical trials, with emerging reports unveiling disturbing details about animal deaths during experiments and allegations of misleading investors about the safety and developmental history of the device.

President Joe Biden’s Department of Justice is asking the Supreme Court to reverse a lower court decision halting two U.S. Food and Drug Administration (FDA) actions that loosened restrictions around mifepristone, the first pill used in a two-drug chemical abortion regimen.

The proportion of telehealth abortions in Colorado is three times the national average of 7.4 percent, according to the report.

The White House lamented a federal court ruling that could block the FDA’s loosened restrictions on mifepristone, a chemical abortion pill.

A federal appeals court issued an order on Wednesday halting two U.S. Food and Drug Administration (FDA) actions which loosened restrictions around mifepristone, the first pill used in a two-drug chemical abortion regimen.

During an interview with Outkick released on Wednesday, 2024 Republican presidential candidate Florida Gov. Ron DeSantis (R) stated that while he wouldn’t want to make Democratic candidate Robert F. Kennedy Jr. his running mate, he would want to “sic him

Women nationwide will no longer need a doctor’s prescription for oral contraceptives — by next year, they will be able to pick them up off the shelves at any drug store.

Sen. Chuck Schumer (D-NY), leader of the Democrat majority in the Senate is targeting YouTube stars Logan Paul and KSI over their energy drink, Prime, which Schumer says has an overly-high caffeine content — despite the fact that a number of other energy drinks have a similar or higher level of caffeine.

Sen. Roger Marshall (R-KS), who is also an obstetrician with more than 25 years of experience, slammed the U.S. Centers for Disease Control and Prevention (CDC) for its guidance advising men who identify as women on how to “chestfeed” infants.

Maryland officials have stockpiled two-and-a-half years’ worth of abortion pills, joining several other Democrat-led states as the nation waits for a decision out of a federal appeals court that could decide the availability of the drug, called mifepristone.
The FDA has granted approval to Elon Musk’s brain implant company, NeuraLink, to carry out clinical studies on humans.

Democrat lawmakers are pushing for insurers to cover over-the-counter birth control, if and when it becomes available without a prescription.

Outside advisers to the U.S. Food and Drug Administration (FDA) voted 17-0 on Wednesday to endorse over-the-counter birth control pills.
The Biden Administration’s mail-order abortion regime is being challenged in part of a federal lawsuit, but no matter the outcome, the USPS plans to “follow the law,” Postmaster General Louis DeJoy says.

Vice President Kamala Harris mistakenly credited a nonexistent federal agency with approving mifepristone, a drug used in a two-drug medication abortion regimen.

Democrat-run Oregon has taken steps to stockpile a three-year supply of the first drug used in a two-drug medication abortion regimen — enough to end the lives of more than 22,000 unborn babies.
